| VT-A |
Pulmonary arterial
hypertension
(PAH) |
|
|
|
|
|
IND - 2024 |
2026
Break-through
Designation |
 -
Pulmonary Arterial Hypertension (PAH)
Pulmonary Arterial Hypertension (PAH) is a rare but fatal disease caused by multiple pathological changes in the pulmonary vasculature. It is characterized by excessive pulmonary vascular remodeling resulting from intimal changes caused by pulmonary artery endothelial cell (PAEC) dysfunction and medial hypertrophy via increased proliferation of pulmonary artery smooth muscle cells (PASMC). This leads to increased pulmonary vascular resistance (PVR) and pulmonary artery pressure, resulting in a marked increase in right ventricular (RV) afterload. Eventually, heart failure develops due to RV hypertrophy.
Currently approved PAH medications are predominantly pulmonary vasodilators: 1) endothelin receptor antagonists, 2) phosphodiesterase type 5 (PDE5) inhibitors, 3) prostacyclin agonists, and 4) soluble guanylyl cyclase (sGC) stimulators. These therapies provide symptomatic relief by primarily inhibiting excessive vasoconstriction and accelerating pulmonary artery vasodilation.
VasThera’s leading drug candidate, VTB-10, has shown promise in reversing pulmonary artery occlusion and ultimately normalizing pulmonary artery structure and function. Mechanistically, VTB-10 inhibits intimal hyperplasia by targeting PDGFR signaling in PASMC while promoting re-endothelialization by restoring VEGFR2 signaling in PAEC.
-

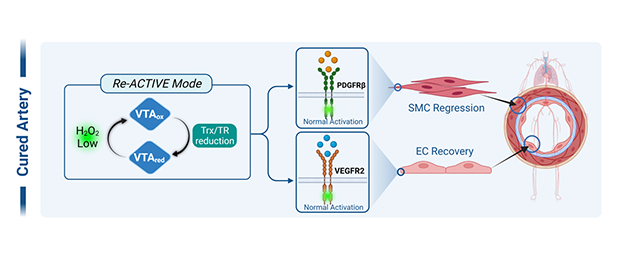
- Choi, M.H., et al. (2005). "Regulation of PDGF signalling and vascular remodeling by peroxiredoxin II." Nature 19:347-35 (total 427).
- Kang, D.H., et al. (2011). "Peroxiredoxin II Is an Essential Antioxidant Enzyme that Prevents the Oxidative Inactivation of VEGF Receptor-2 in Vascular Endothelial Cells." Molecular Cell 44:545-558 (total 116).
- Kang, D.H., et al. (2013). "Vascular injury involves the overoxidation of peroxiredoxin type II and is recovered by the peroxiredoxin-activity mimetic that induces reendothelialization." Circulation 128(8):834-844 (total 21).
|
 -
Pulmonary Arterial Hypertension (PAH)
Pulmonary Arterial Hypertension (PAH) is a rare but fatal disease caused by multiple pathological changes in the pulmonary vasculature. It is characterized by excessive pulmonary vascular remodeling resulting from intimal changes caused by pulmonary artery endothelial cell (PAEC) dysfunction and medial hypertrophy via increased proliferation of pulmonary artery smooth muscle cells (PASMC). This leads to increased pulmonary vascular resistance (PVR) and pulmonary artery pressure, resulting in a marked increase in right ventricular (RV) afterload. Eventually, heart failure develops due to RV hypertrophy.
Currently approved PAH medications are predominantly pulmonary vasodilators: 1) endothelin receptor antagonists, 2) phosphodiesterase type 5 (PDE5) inhibitors, 3) prostacyclin agonists, and 4) soluble guanylyl cyclase (sGC) stimulators. These therapies provide symptomatic relief by primarily inhibiting excessive vasoconstriction and accelerating pulmonary artery vasodilation.
VasThera’s leading drug candidate, VTB-10, has shown promise in reversing pulmonary artery occlusion and ultimately normalizing pulmonary artery structure and function. Mechanistically, VTB-10 inhibits intimal hyperplasia by targeting PDGFR signaling in PASMC while promoting re-endothelialization by restoring VEGFR2 signaling in PAEC.
-


- Choi, M.H., et al. (2005). "Regulation of PDGF signalling and vascular remodeling by peroxiredoxin II." Nature 19:347-35 (total 427).
- Kang, D.H., et al. (2011). "Peroxiredoxin II Is an Essential Antioxidant Enzyme that Prevents the Oxidative Inactivation of VEGF Receptor-2 in Vascular Endothelial Cells." Molecular Cell 44:545-558 (total 116).
- Kang, D.H., et al. (2013). "Vascular injury involves the overoxidation of peroxiredoxin type II and is recovered by the peroxiredoxin-activity mimetic that induces reendothelialization." Circulation 128(8):834-844 (total 21).
|
| VT-C |
Triple-negative
breast cancer
(TNBC) |
|
|
|
|
|
|
|
 -
Triple Negative Breast Cancer (TNBC)
Breast cancer was the leading cause of cancer death among women worldwide in 2018. It is classified into four major molecular subtypes: luminal A, luminal B, basal-like/triple-negative/basal breast cancer(TNBC)-negative, and HER2. TNBC, characterized by little or no expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2), exhibits highly metastatic and recurrent behaviors. Targeting the eradication of TNBC is crucial for improving the relapse-free survival of BC patients.
EGFR, a receptor tyrosine kinase (RTK) member of the HER family, plays a key role in regulating cancer cell growth and metabolism. EGF, a ligand for EGFR, is known to enhance glucose consumption and lactate production in cancer cells. In particular, EGFR overexpression is frequently observed in TNBC patients. Thus, EGFR represents a promising new therapeutic target for treating TNBC patients.
Our anti-TNBC drug candidate, VTA-04, was developed using the RedoxizymeTM platform. Specifically designed for TNBC, VTA-04 induces EGFR-dependent reprogramming of energy metabolism, effectively inhibiting cancer cell proliferation and metastasis.
- Lee, E., et al. (2020). "Glutathione peroxidase-1 regulates adhesion and metastasis of triplenegative breast cancer cells via FAK signaling." Redox Biology 29.
- McLaughlin, R. P., et al. (2019). "A kinase inhibitor screen identifies a dual cdc7/CDK9 inhibitor to sensitise triple-negative breast cancer to EGFR-targeted therapy." Breast Cancer Res 21(1): 77.
- Shen, M., et al. (2019). "Tinagl1 Suppresses TripleNegative Breast Cancer Progression and Metastasis by Simultaneously Inhibiting Integrin/FAK and EGFR Signaling." Cancer Cell 35(1): 64-80 e67.
- Wendt, M. K., et al. (2017). "The paradoxical functions of EGFR during breast cancer progression." Signal Transduction and Targeted Therapy 2:16042.
|
 -
Triple Negative Breast Cancer (TNBC)
Breast cancer was the leading cause of cancer death among women worldwide in 2018. It is classified into four major molecular subtypes: luminal A, luminal B, basal-like/triple-negative/basal breast cancer(TNBC)-negative, and HER2. TNBC, characterized by little or no expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2), exhibits highly metastatic and recurrent behaviors. Targeting the eradication of TNBC is crucial for improving the relapse-free survival of BC patients.
EGFR, a receptor tyrosine kinase (RTK) member of the HER family, plays a key role in regulating cancer cell growth and metabolism. EGF, a ligand for EGFR, is known to enhance glucose consumption and lactate production in cancer cells. In particular, EGFR overexpression is frequently observed in TNBC patients. Thus, EGFR represents a promising new therapeutic target for treating TNBC patients.
Our anti-TNBC drug candidate, VTA-04, was developed using the RedoxizymeTM platform. Specifically designed for TNBC, VTA-04 induces EGFR-dependent reprogramming of energy metabolism, effectively inhibiting cancer cell proliferation and metastasis.
- Lee, E., et al. (2020). "Glutathione peroxidase-1 regulates adhesion and metastasis of triplenegative breast cancer cells via FAK signaling." Redox Biology 29.
- McLaughlin, R. P., et al. (2019). "A kinase inhibitor screen identifies a dual cdc7/CDK9 inhibitor to sensitise triple-negative breast cancer to EGFR-targeted therapy." Breast Cancer Res 21(1): 77.
- Shen, M., et al. (2019). "Tinagl1 Suppresses TripleNegative Breast Cancer Progression and Metastasis by Simultaneously Inhibiting Integrin/FAK and EGFR Signaling." Cancer Cell 35(1): 64-80 e67.
- Wendt, M. K., et al. (2017). "The paradoxical functions of EGFR during breast cancer progression." Signal Transduction and Targeted Therapy 2:16042.
|
| VT-N |
Alzheimer's disease
(AD) |
|
|
|
|
|
|
|

-
Alzheimer's Disease (AD)
Alzheimer's disease (AD) is the most common form of dementia characterized by cognitive dysfunction resulting from neuronal loss. The hallmarks of AD include senile plaques and neurofibrillary tangles. The accumulation of β-amyloid (Aβ) and p-tau contributes to neuronal death through oxidative stress, leading to neuroinflammation and synaptic dysfunction. Recently, antibody drugs targeting amyloid plaques have shown promising therapeutic efficacy in early-stage Alzheimer's disease.
At VasThera, we are dedicated to developing orally administered drugs in small molecule designed to inhibit both astrocyte activation and neuronal death. In preclinical studies using an Alzheimer's mouse model, our drug candidate, VTA-27, has exhibited robust efficacy in clearing amyloid plaques and restoring cognitive behavior.
- Alzheimer's Association. (2012). "Alzheimer's disease facts and figures." Alzheimers Dement 8, 131-168.
- Querfurth, H.W. (2010). "Alzheimer's disease." The New Eng J of Med 362, 329-344.
- Cheignin, C et al. (2018). "Oxidative stress and the amyloid beta peptide in Alzheimer's disease." Redox Biol 14, 450-464.
- Hardy J, Selkoe DJ. (2002). "The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics." Science 297, 353-356.
- Chun H et al. (2020). "Severe reactive astrocytes precipitate pathological hallmarks of AD via H2O2- production." Nat. neurosci 23, 1555-1566.
|

-
Alzheimer's Disease (AD)
Alzheimer's disease (AD) is the most common form of dementia characterized by cognitive dysfunction resulting from neuronal loss. The hallmarks of AD include senile plaques and neurofibrillary tangles. The accumulation of β-amyloid (Aβ) and p-tau contributes to neuronal death through oxidative stress, leading to neuroinflammation and synaptic dysfunction. Recently, antibody drugs targeting amyloid plaques have shown promising therapeutic efficacy in early-stage Alzheimer's disease.
At VasThera, we are dedicated to developing orally administered drugs in small molecule designed to inhibit both astrocyte activation and neuronal death. In preclinical studies using an Alzheimer's mouse model, our drug candidate, VTA-27, has exhibited robust efficacy in clearing amyloid plaques and restoring cognitive behavior.
- Alzheimer's Association. (2012). "Alzheimer's disease facts and figures." Alzheimers Dement 8, 131-168.
- Querfurth, H.W. (2010). "Alzheimer's disease." The New Eng J of Med 362, 329-344.
- Cheignin, C et al. (2018). "Oxidative stress and the amyloid beta peptide in Alzheimer's disease." Redox Biol 14, 450-464.
- Hardy J, Selkoe DJ. (2002). "The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics." Science 297, 353-356.
- Chun H et al. (2020). "Severe reactive astrocytes precipitate pathological hallmarks of AD via H2O2- production." Nat. neurosci 23, 1555-1566.
|
| VT-E |
Age-related macular
degeneration
(AMD) |
|
|
|
|
|
|
|
-

-
Age-related Macular Degeneration (AMD)
Wet AMD involves abnormal blood vessels around the macula and retina, leading to rapid and severe vision loss. Anti-VEGFA therapies available for wet AMD have demonstrated clinical success.
Dry AMD affects approximately 80-90% of people with AMD, and vision loss typically progresses gradually and it is clinically defined by the progressive accumulation of drusen and pigmentary abnormalities in the retinal pigment epithelium (RPE).
The most critical function of RPE is the daily phagocytosis and clearance of photoreceptor outer segments through the autophagic process. Consequently, dry AMD is characterized by the accumulation of intracellular lipofuscin and extracellular drusen due to autophagy pathway impairment. In particular, the loss of lysosomal activity in the RPE associated with aging exacerbates alterations in autophagy, potentially aggravating visual function.
The FDA has approved the first drugs to treat dry AMD, Syfovre (Apellis Pharmaceutical) and Izervay (Iveric Bio), which are complement inhibitors. However, the exact mechanisms underlying the multifactorial etiology of dry AMD remain unclear, so that there are still no effective treatment protocols.
Our Opti-ATG platform can develop novel chemical candidates designed to optimize the autophagic flux and lysosomal pathway in dry AMD, ultimately restoring the viability and function of RPE cells.
- Bradley, D. G., et al. (2016). “A Revised Hemodynamic Theory of Age-Related Macular Degeneration” Trends in Molecular Medicine 22(8): 656-670.
- Li, T. T., et al. (2020). “Complement activation, lipid metabolism, and mitochondrial injury: Converging pathways in age-related macular degeneration” Redox Biology 37: 101781-.
- Monika, F., et al. (2021). “Age-related macular degeneration” Nature reviews disease primers 7(31): 1-25.
|
-

-
Age-related Macular Degeneration (AMD)
Wet AMD involves abnormal blood vessels around the macula and retina, leading to rapid and severe vision loss. Anti-VEGFA therapies available for wet AMD have demonstrated clinical success.
Dry AMD affects approximately 80-90% of people with AMD, and vision loss typically progresses gradually and it is clinically defined by the progressive accumulation of drusen and pigmentary abnormalities in the retinal pigment epithelium (RPE).
The most critical function of RPE is the daily phagocytosis and clearance of photoreceptor outer segments through the autophagic process. Consequently, dry AMD is characterized by the accumulation of intracellular lipofuscin and extracellular drusen due to autophagy pathway impairment. In particular, the loss of lysosomal activity in the RPE associated with aging exacerbates alterations in autophagy, potentially aggravating visual function.
The FDA has approved the first drugs to treat dry AMD, Syfovre (Apellis Pharmaceutical) and Izervay (Iveric Bio), which are complement inhibitors. However, the exact mechanisms underlying the multifactorial etiology of dry AMD remain unclear, so that there are still no effective treatment protocols.
Our Opti-ATG platform can develop novel chemical candidates designed to optimize the autophagic flux and lysosomal pathway in dry AMD, ultimately restoring the viability and function of RPE cells.
- Bradley, D. G., et al. (2016). “A Revised Hemodynamic Theory of Age-Related Macular Degeneration” Trends in Molecular Medicine 22(8): 656-670.
- Li, T. T., et al. (2020). “Complement activation, lipid metabolism, and mitochondrial injury: Converging pathways in age-related macular degeneration” Redox Biology 37: 101781-.
- Monika, F., et al. (2021). “Age-related macular degeneration” Nature reviews disease primers 7(31): 1-25.
|

 Technology
Technology Pipeline
Pipeline








 TOP
TOP